Water Freezes At What

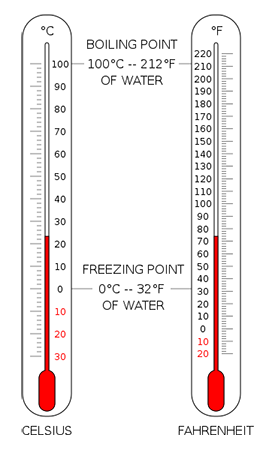
Water freezing is a fundamental physical process that occurs when the temperature of water is lowered to a certain point. At standard atmospheric pressure, water freezes at a temperature of 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F). This temperature is also known as the freezing point of water. The freezing point of water is a well-defined physical constant that is used as a reference point in many scientific and engineering applications.
Thermodynamic Properties of Water Freezing
The process of water freezing involves a change in the state of matter from liquid to solid, which is accompanied by a release of heat energy. The amount of heat energy released during freezing is known as the latent heat of fusion, which is approximately 334 joules per gram (J/g) for water. The freezing process also involves a change in the crystal structure of water, with the formation of a crystalline solid known as ice.
Factors Affecting the Freezing Point of Water
The freezing point of water can be affected by several factors, including pressure, salinity, and dissolved gases. At higher pressures, the freezing point of water is lowered, which is why water can remain in a liquid state at temperatures below 0°C under high pressure. The presence of dissolved salts or other substances can also lower the freezing point of water, which is why seawater typically freezes at a temperature below 0°C. Additionally, the presence of dissolved gases, such as air, can affect the freezing point of water by introducing nucleation sites that can facilitate the formation of ice crystals.
| Factor | Effect on Freezing Point |
|---|---|
| Increased Pressure | Lowering of freezing point |
| Salinity | Lowering of freezing point |
| Dissolved Gases | Introduction of nucleation sites |

Applications of Water Freezing

The process of water freezing has numerous practical applications in various fields, including refrigeration, food processing, and environmental science. In refrigeration, the freezing of water is used to cool foods and beverages to a safe temperature for storage and transportation. In food processing, freezing is used to preserve fruits, vegetables, and meats by preventing the growth of microorganisms and reducing enzymatic activity. In environmental science, the freezing of water is an important factor in climate modeling and weather forecasting, as it affects the formation of ice sheets, glaciers, and sea ice.
Future Implications of Water Freezing Research
Research on the freezing of water is ongoing, with a focus on understanding the underlying physical and chemical processes that govern this complex phenomenon. Future studies are likely to explore the effects of climate change on the freezing point of water, as well as the development of new technologies for freezing and preserving water. Additionally, researchers are investigating the potential applications of supercooling and flash freezing in various fields, including food preservation and biomedical engineering.
- Climate change: Understanding the effects of climate change on the freezing point of water and its implications for environmental science and policy.
- Technological innovations: Developing new technologies for freezing and preserving water, including advanced refrigeration systems and novel freezing methods.
- Food preservation: Investigating the potential applications of supercooling and flash freezing in food preservation, including the development of new frozen food products and technologies.
What is the freezing point of water at standard atmospheric pressure?
+The freezing point of water at standard atmospheric pressure is 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F).
What factors can affect the freezing point of water?
+The freezing point of water can be affected by several factors, including pressure, salinity, and dissolved gases.
What are some practical applications of water freezing?
+The process of water freezing has numerous practical applications in various fields, including refrigeration, food processing, and environmental science.