Where Is The Electron Located In The Atom

The location of electrons in an atom is a fundamental concept in physics and chemistry. The atom is the basic building block of matter, and understanding the structure of atoms is crucial for understanding the behavior of matter at the atomic and molecular level. The electron is a subatomic particle that carries a negative charge and is one of the three main components of an atom, along with protons and neutrons.
The Atomic Structure

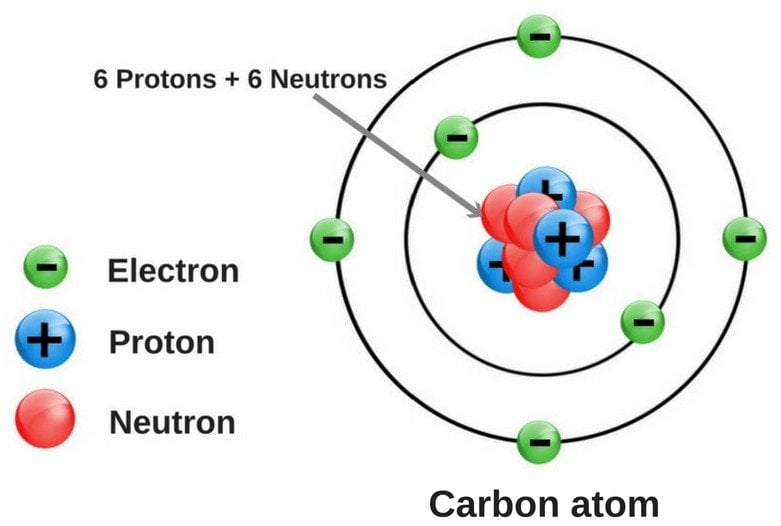
The atom is composed of a nucleus, which is the central part of the atom, and the electrons, which orbit around the nucleus. The nucleus is made up of protons and neutrons, which are collectively known as nucleons. The protons have a positive charge, while the neutrons are neutral. The electrons, on the other hand, have a negative charge and are attracted to the positively charged protons in the nucleus.
The electrons are located in a region around the nucleus known as the electron cloud or atomic orbital. The electron cloud is a three-dimensional region where the electrons are likely to be found. The shape and size of the electron cloud depend on the energy level of the electron and the type of atomic orbital. The atomic orbital is a mathematical description of the probability of finding an electron within a particular region around the nucleus.
Energy Levels and Electron Shells
The electrons in an atom are arranged in energy levels or electron shells. Each energy level can hold a specific number of electrons, and the electrons in each energy level have a specific amount of energy. The energy levels are typically labeled with the letters K, L, M, N, O, P, Q, and so on, with the K shell being the closest to the nucleus. The electrons in each energy level are arranged in subshells, which are further divided into orbitals.
The number of electrons in each energy level is determined by the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. The quantum numbers describe the energy, shape, and orientation of the electron orbital. The energy levels and electron shells are filled in a specific order, with the lowest energy levels being filled first.
| Energy Level | Electron Capacity |
|---|---|
| K shell | 2 electrons |
| L shell | 8 electrons |
| M shell | 18 electrons |
| N shell | 32 electrons |
| O shell | 50 electrons |
Electron Location and Probability

The location of an electron in an atom is described by a probability distribution. The probability distribution is a mathematical function that describes the likelihood of finding an electron within a particular region around the nucleus. The probability distribution is typically represented by a radial probability distribution or a probability density.
The radial probability distribution describes the probability of finding an electron at a given distance from the nucleus. The probability density, on the other hand, describes the probability of finding an electron within a given volume element around the nucleus. The probability distribution is used to calculate the expectation value of the electron's position, which is the average position of the electron in the atom.
Heisenberg Uncertainty Principle
The Heisenberg uncertainty principle states that it is impossible to know the exact position and momentum of an electron at the same time. The uncertainty principle is a fundamental concept in quantum mechanics and is a result of the wave-particle duality of electrons. The uncertainty principle is mathematically represented by the uncertainty relation, which describes the relationship between the uncertainty in position and momentum.
The uncertainty principle has important implications for the location of electrons in an atom. It means that the position of an electron cannot be precisely known, and the electron's position is described by a probability distribution. The uncertainty principle is a fundamental aspect of quantum mechanics and is essential for understanding the behavior of electrons in atoms and molecules.
What is the electron cloud?
+The electron cloud is a three-dimensional region around the nucleus where the electrons are likely to be found. It is a mathematical description of the probability of finding an electron within a particular region around the nucleus.
What is the Pauli exclusion principle?
+The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. It determines the number of electrons in each energy level and the arrangement of electrons in an atom.
In conclusion, the location of electrons in an atom is described by a probability distribution, which is a mathematical function that describes the likelihood of finding an electron within a particular region around the nucleus. The electron cloud, energy levels, and electron shells are all important concepts in understanding the structure of atoms and the behavior of electrons. The Heisenberg uncertainty principle and the Pauli exclusion principle are fundamental aspects of quantum mechanics that have important implications for the location of electrons in an atom.



