Periodic Table Of Elements With Molar Mass
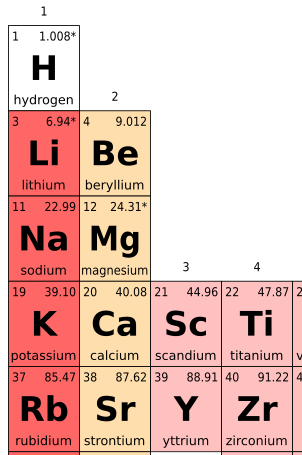
The periodic table of elements is a tabular arrangement of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. The periodic table provides a framework for understanding the properties and behavior of elements, including their molar mass, which is the mass of one mole of an element's atoms.
Introduction to the Periodic Table
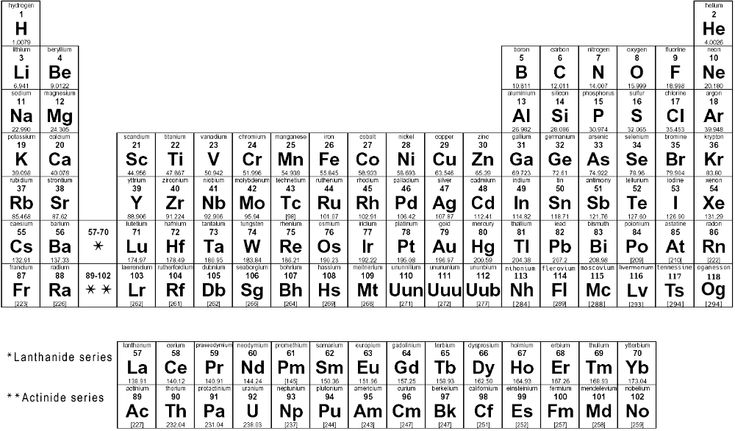
The periodic table is a powerful tool for chemists, physicists, and other scientists, as it allows them to predict the properties and behavior of elements based on their position in the table. The elements are arranged in a logical and systematic way, with elements that exhibit similar properties and behavior being placed in the same group or family. The periodic table also provides a way to identify the atomic mass of an element, which is the total number of protons and neutrons in the nucleus of an atom.
Understanding Molar Mass
Molar mass is a measure of the mass of one mole of an element’s atoms. It is expressed in units of grams per mole (g/mol) and is calculated by summing the masses of the protons, neutrons, and electrons in a single atom of the element. The molar mass of an element is an important property, as it is used to calculate the number of moles of an element in a given sample, as well as the mass of a sample in grams.
The following table lists the elements of the periodic table, along with their atomic number, symbol, and molar mass.
| Atomic Number | Symbol | Molar Mass (g/mol) |
|---|---|---|
| 1 | H | 1.00794 |
| 2 | He | 4.002602 |
| 3 | Li | 6.941 |
| 4 | Be | 9.012182 |
| 5 | B | 10.811 |
| 6 | C | 12.0107 |
| 7 | N | 14.0067 |
| 8 | O | 15.9994 |
| 9 | F | 18.9984032 |
| 10 | Ne | 20.1797 |
| 11 | Na | 22.98976928 |
| 12 | Mg | 24.305 |
| 13 | Al | 26.9815385 |
| 14 | Si | 28.0855 |
| 15 | P | 30.973762 |
| 16 | S | 32.064 |
| 17 | Cl | 35.453 |
| 18 | Ar | 39.9483 |
| 19 | K | 39.0983 |
| 20 | Ca | 40.078 |
| 21 | Sc | 44.955907 |
| 22 | Ti | 47.867 |
| 23 | V | 50.9415 |
| 24 | Cr | 51.9961 |
| 25 | Mn | 54.938044 |
| 26 | Fe | 55.847 |
| 27 | Co | 58.933194 |
| 28 | Ni | 58.6934 |
| 29 | Cu | 63.546 |
| 30 | Zn | 65.38 |
| 31 | Ga | 69.723 |
| 32 | Ge | 72.59 |
| 33 | As | 74.92159 |
| 34 | Se | 78.96 |
| 35 | Br | 79.904 |
| 36 | Kr | 83.798 |
| 37 | Rb | 85.4678 |
| 38 | Sr | 87.62 |
| 39 | Y | 88.90585 |
| 40 | Zr | 91.224 |
| 41 | Nb | 92.90638 |
| 42 | Mo | 95.95 |
| 43 | Tc | 98 |
| 44 | Ru | 101.07 |
| 45 | Rh | 102.9055 |
| 46 | Pd | 106.42 |
| 47 | Ag | 107.8682 |
| 48 | Cd | 112.414 |
| 49 | In | 114.818 |
| 50 | Sn | 118.71 |
| 51 | Sb | 121.76 |
| 52 | Te | 127.6 |
| 53 | I | 126.90447 |
| 54 | Xe | 131.293 |
| 55 | Cs | 132.9133 |
| 56 | Ba | 137.327 |
| 57 | La | 138.90547 |
| 58 | Ce | 140.116 |
| 59 | Pr | 140.90765 |
| 60 | Nd | 144.242 |
| 61 | Pm | 145 |
| 62 | Sm | 150.36</ |