Periodic Table Of Elements With Electron Configuration
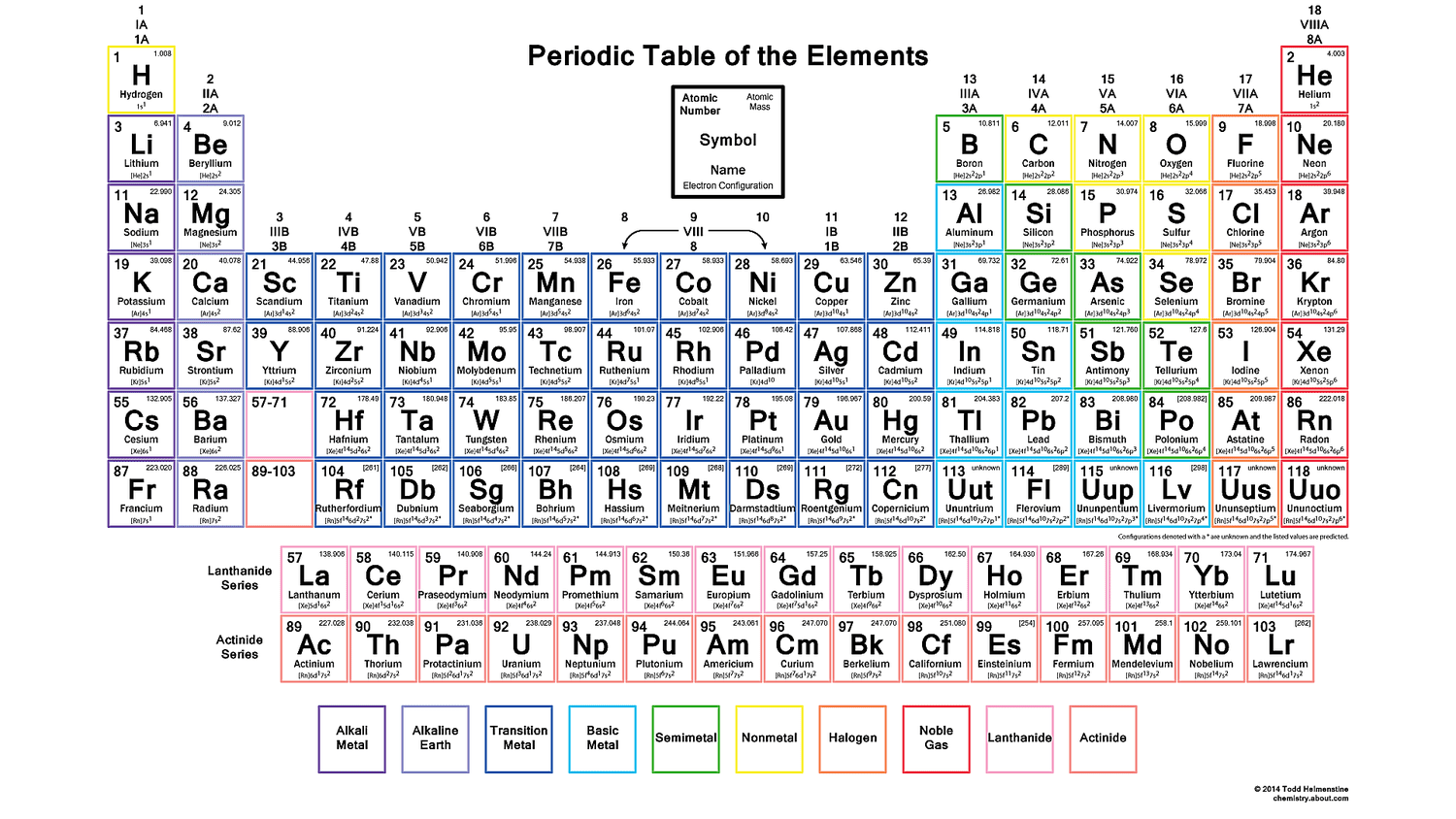
The periodic table of elements is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. The periodic table is a powerful tool for predicting the properties and behavior of elements, and it has played a crucial role in the development of modern chemistry.
Introduction to Electron Configuration
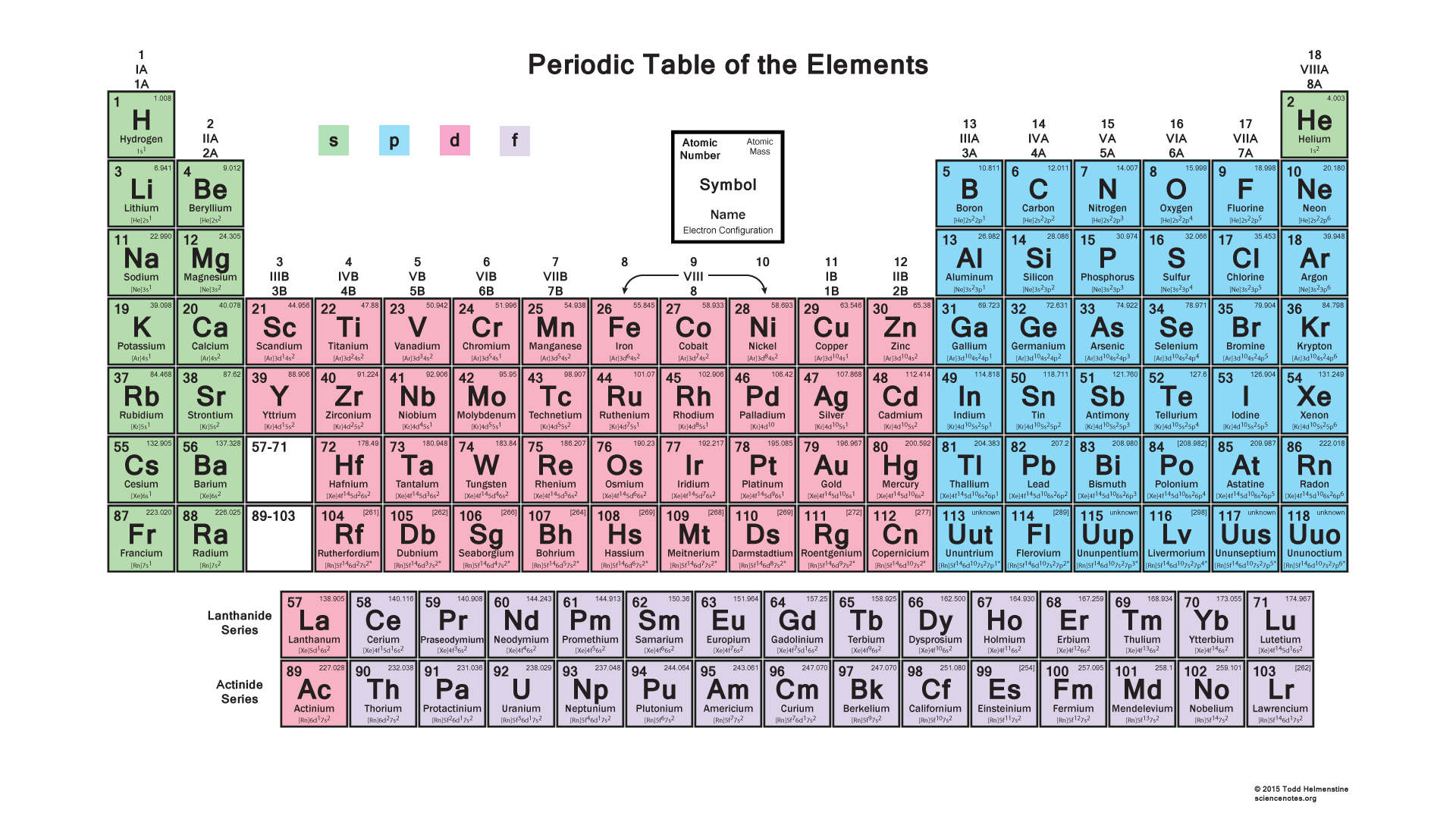
Electron configuration is the distribution of electrons in an atom, which determines the chemical properties of an element. The electron configuration of an atom is typically written in a shorthand notation, with the number of electrons in each energy level (or shell) and the number of electrons in each orbital. The Aufbau principle and the Pauli exclusion principle are used to determine the electron configuration of an atom. The Aufbau principle states that electrons occupy the lowest available energy levels, while the Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers.
Understanding Electron Configuration Notation
The electron configuration notation is a shorthand way of describing the distribution of electrons in an atom. It consists of a series of numbers and letters that indicate the energy level, orbital, and number of electrons in each orbital. For example, the electron configuration of hydrogen is 1s1, which means that there is one electron in the 1s orbital. The electron configuration of helium is 1s2, which means that there are two electrons in the 1s orbital.
The following is a breakdown of the electron configuration notation:
- s-orbitals: can hold up to 2 electrons
- p-orbitals: can hold up to 6 electrons
- d-orbitals: can hold up to 10 electrons
- f-orbitals: can hold up to 14 electrons
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen | 1 | 1s1 |
| Helium | 2 | 1s2 |
| Lithium | 3 | 1s2 2s1 |
| Beryllium | 4 | 1s2 2s2 |
| Boron | 5 | 1s2 2s2 2p1 |

Blocks of the Periodic Table
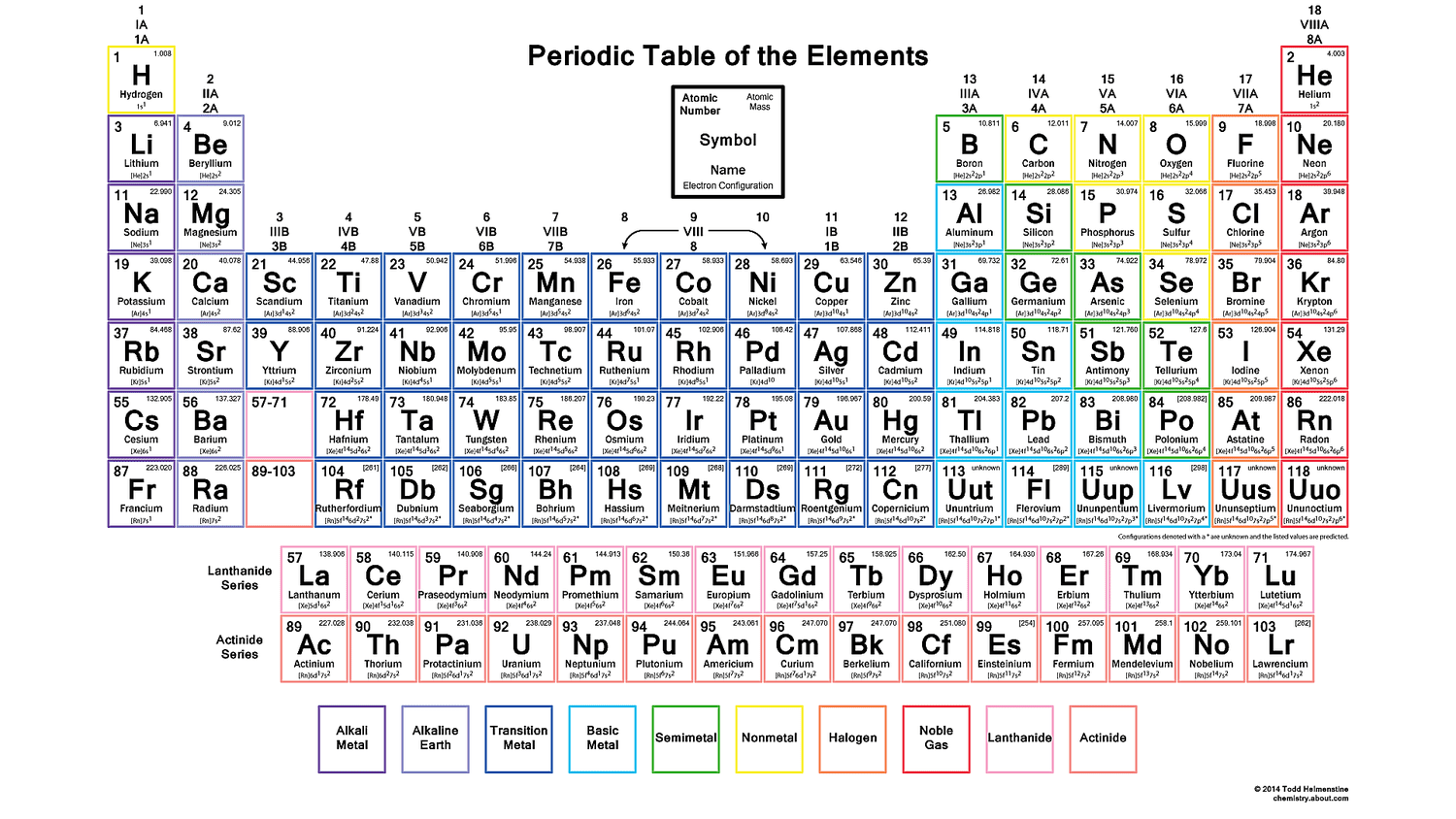
The periodic table is divided into several blocks, each of which corresponds to a specific type of orbital. The s-block elements are in the first two columns of the periodic table and have an s-orbital in their outermost energy level. The p-block elements are in the last six columns of the periodic table and have a p-orbital in their outermost energy level. The d-block elements are in the middle of the periodic table and have a d-orbital in their outermost energy level. The f-block elements are at the bottom of the periodic table and have an f-orbital in their outermost energy level.
Properties of the Blocks
Each block of the periodic table has its own unique properties and characteristics. The s-block elements are typically metals and are highly reactive. The p-block elements are nonmetals or metalloids and are less reactive than the s-block elements. The d-block elements are transition metals and are known for their ability to form ions with different charges. The f-block elements are lanthanides and actinides and are highly reactive and toxic.
The following is a summary of the properties of the blocks:
- s-block: highly reactive, typically metals
- p-block: less reactive, nonmetals or metalloids
- d-block: transition metals, can form ions with different charges
- f-block: highly reactive, toxic, lanthanides and actinides
What is the importance of electron configuration in chemistry?
+Electron configuration is important in chemistry because it determines the chemical properties and behavior of an element. By understanding the electron configuration of an element, chemists can predict its reactivity, identify its ions, and determine its position in the periodic table.
How do the blocks of the periodic table differ from each other?
+The blocks of the periodic table differ from each other in terms of the type of orbital in their outermost energy level. The s-block elements have an s-orbital, the p-block elements have a p-orbital, the d-block elements have a d-orbital, and the f-block elements have an f-orbital. Each block has its own unique properties and characteristics, such as reactivity and toxicity.
In conclusion, the periodic table of elements is a powerful tool for predicting the properties and behavior of elements, and electron configuration plays a crucial role in determining the chemical properties of an element. By understanding the electron configuration of an element and the blocks of the periodic table, chemists can predict the reactivity and other properties of an element and identify its position in the periodic table.