O Atomic Mass: Understand Elements
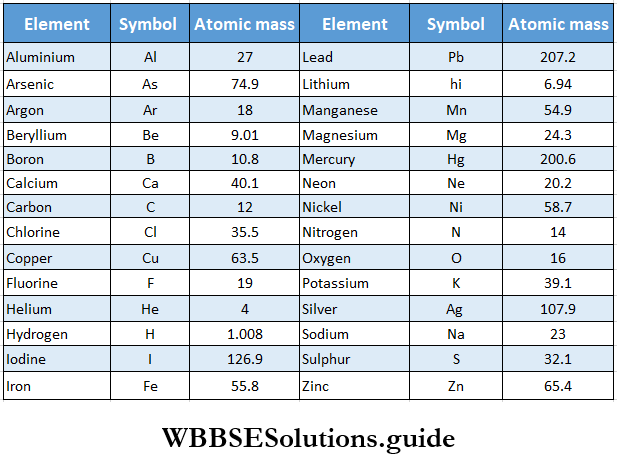
The concept of atomic mass is fundamental in understanding the properties and behavior of elements. Atomic mass, also known as atomic weight, is the total mass of an atom, which includes the masses of its protons, neutrons, and electrons. However, since the mass of electrons is negligible compared to that of protons and neutrons, atomic mass is primarily determined by the sum of the masses of protons and neutrons in the nucleus. This understanding is crucial for chemists and physicists as it underpins the periodic table and the classification of elements.
Introduction to Atomic Mass
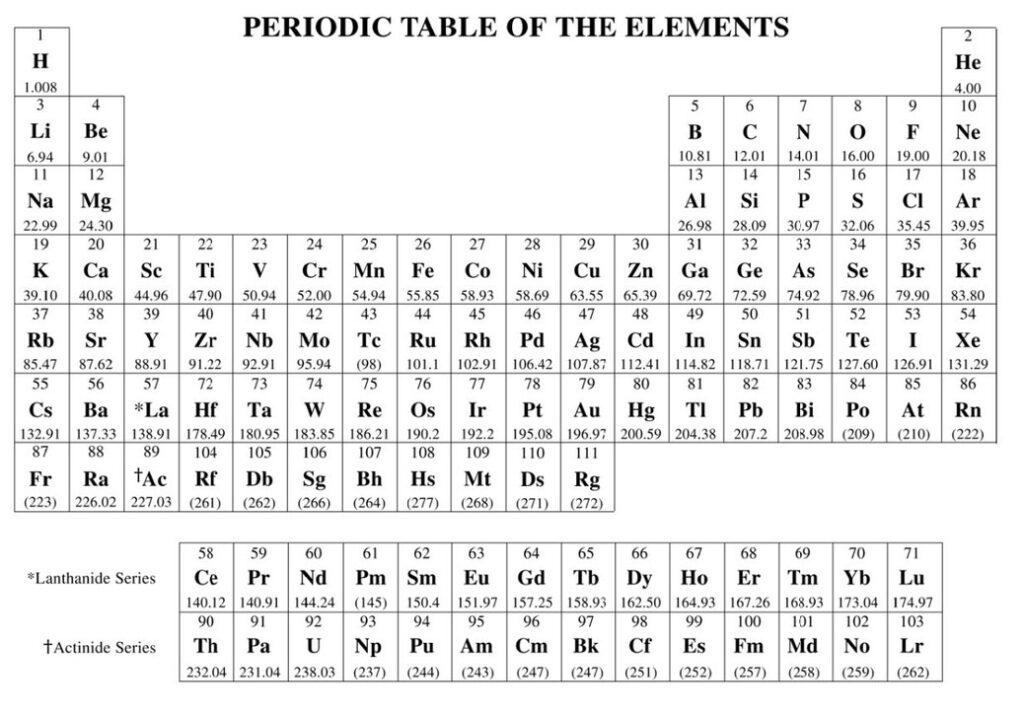
Atomic mass is measured in atomic mass units (amu), also known as unified atomic mass units (u). One atomic mass unit is defined as one-twelfth the mass of a carbon-12 atom. This standardization allows for the precise comparison of the masses of different atoms. The atomic mass of an element is not an integer because it represents the average mass of the naturally occurring isotopes of that element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons, leading to variations in mass.
Isotopes and Atomic Mass
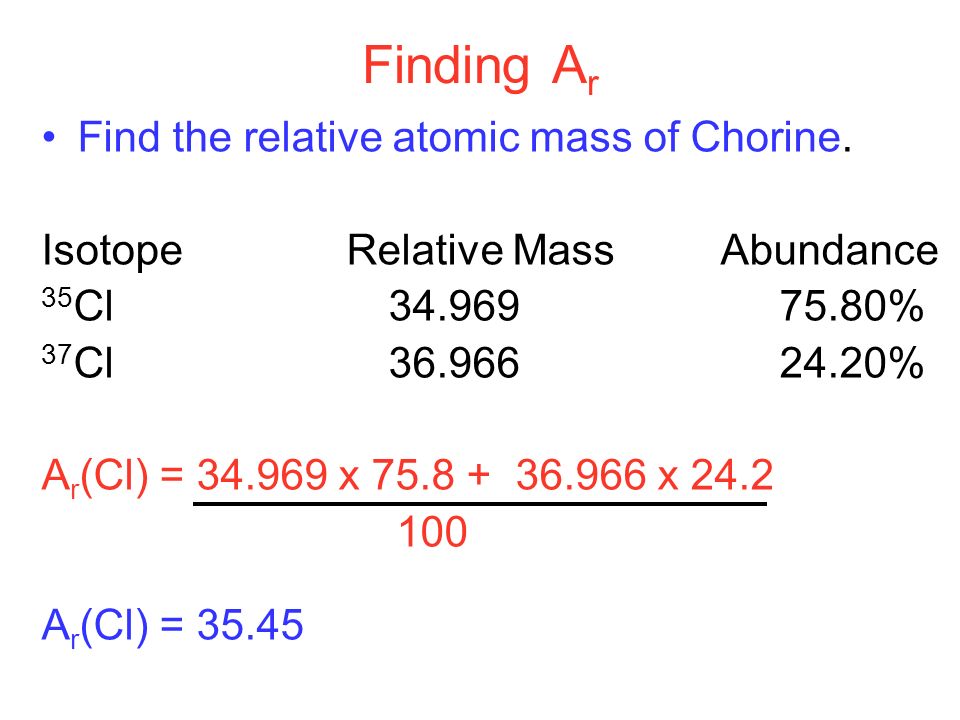
The existence of isotopes means that the atomic mass of an element is an average value, calculated based on the masses and relative abundances of its naturally occurring isotopes. For instance, chlorine has two main isotopes: chlorine-35 and chlorine-37, with masses of approximately 35 u and 37 u, respectively. The average atomic mass of chlorine, which takes into account the relative abundance of these isotopes, is approximately 35.5 u. This averaging is what makes the atomic masses of elements non-integer values.
| Isotope | Mass (u) | Relative Abundance |
|---|---|---|
| Chlorine-35 | 34.9689 | 75.78% |
| Chlorine-37 | 36.9659 | 24.22% |
Calculation of Atomic Mass
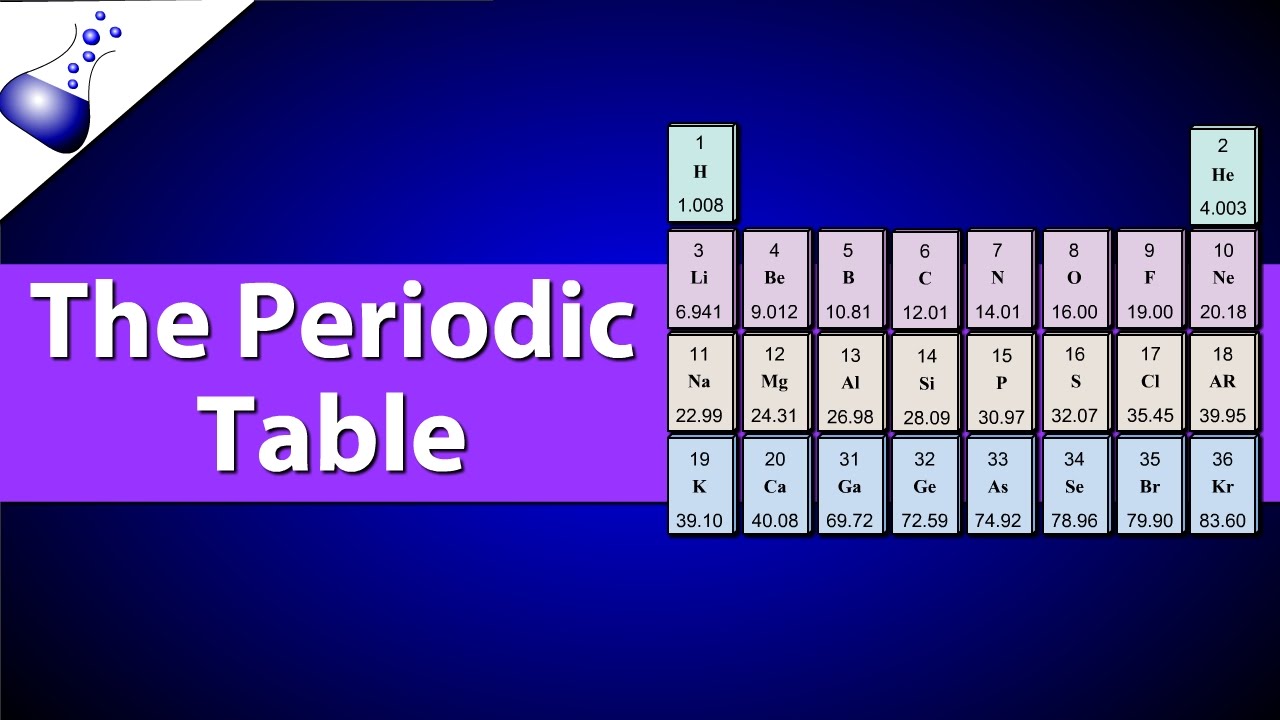
The calculation of atomic mass involves knowing the masses of the isotopes of an element and their relative abundances in nature. The atomic mass (A) of an element can be calculated using the formula: A = (mass of isotope 1 * abundance of isotope 1) + (mass of isotope 2 * abundance of isotope 2) + …, where the abundance is expressed as a fraction or decimal of the total. This formula illustrates how the atomic mass reflects the average properties of the isotopes of an element.
Importance of Atomic Mass in Chemistry
In chemistry, atomic mass is crucial for calculating the molar mass of compounds, which is essential for stoichiometry—the part of chemistry that studies amounts of substances that are involved in reactions. Knowing the atomic masses of elements allows chemists to calculate the molar masses of compounds and predict the amounts of reactants needed and products formed in chemical reactions. This is fundamental for both theoretical calculations and practical applications in synthesis, analysis, and industrial production.
The atomic mass also plays a critical role in understanding the periodic trends and properties of elements. Elements with similar atomic masses but different atomic numbers (number of protons) can exhibit different chemical properties due to differences in their electron configurations. The arrangement of electrons around the nucleus, influenced by the atomic mass and atomic number, determines the chemical reactivity and physical properties of an element.
| Element | Atomic Number | Atomic Mass (u) |
|---|---|---|
| Hydrogen | 1 | 1.00794 |
| Helium | 2 | 4.002602 |
| Lithium | 3 | 6.941 |
What is the significance of atomic mass in understanding elements?
+Atomic mass is significant because it allows for the identification and classification of elements, understanding of isotopic variations, and calculation of molar masses of compounds, which are crucial in chemistry and physics for predicting properties and behaviors of substances.
How are atomic masses calculated for elements with multiple isotopes?
+Atomic masses for elements with multiple isotopes are calculated by averaging the masses of the naturally occurring isotopes, weighted by their relative abundances. This reflects the average properties of the element as found in nature.
In conclusion, the concept of atomic mass is central to the understanding of elements and their properties. It is a fundamental principle in chemistry and physics, underpinning our ability to classify elements, understand their isotopic variations, and predict their chemical and physical behaviors. The calculation of atomic mass, which takes into account the masses and abundances of isotopes, provides a quantitative basis for these understandings, highlighting the importance of precise measurement and calculation in scientific inquiry.

