Is Salt A Compound

Salt, commonly referred to as table salt, is primarily composed of sodium chloride (NaCl), which is indeed a compound. A compound is a substance formed when two or more different elements are chemically bonded together. In the case of sodium chloride, it consists of one sodium (Na) atom and one chlorine (Cl) atom, making it a binary compound.
Chemical Composition of Salt
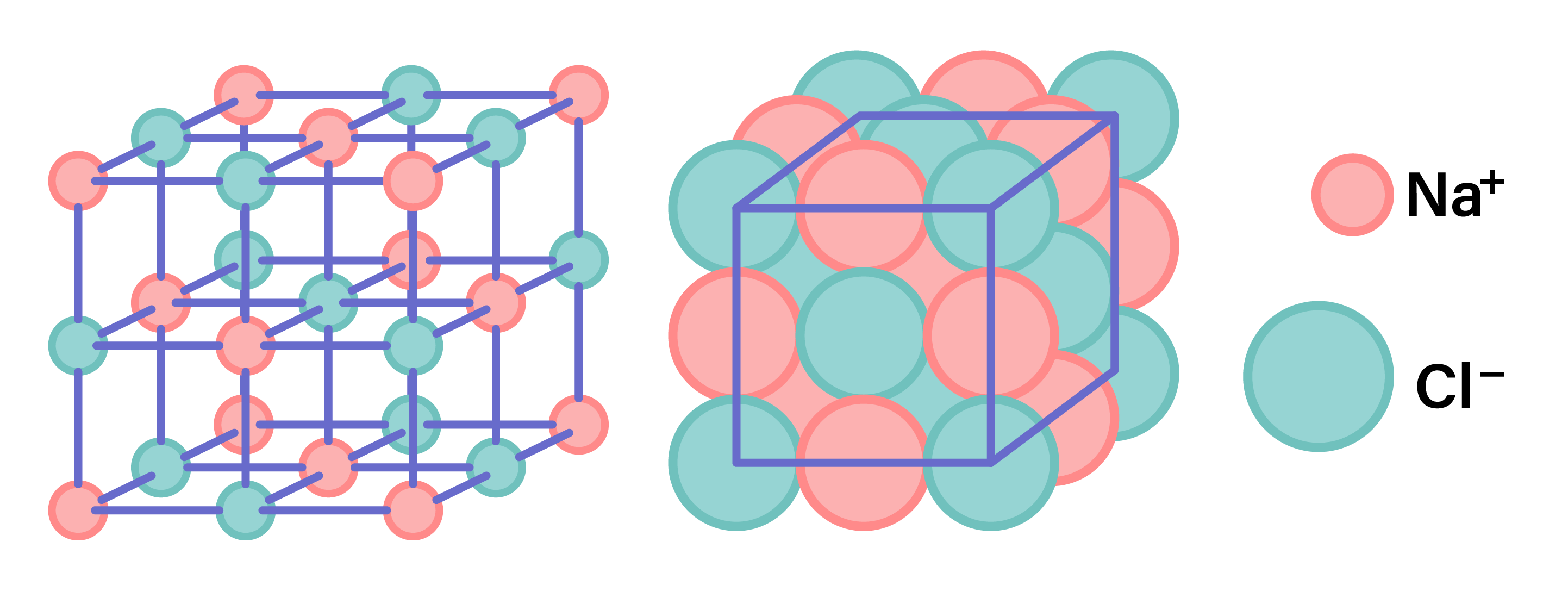
The chemical formula for sodium chloride, NaCl, signifies that one atom of sodium combines with one atom of chlorine to form a molecule of salt. This chemical bonding is a result of the transfer of electrons from the sodium atom to the chlorine atom, resulting in the formation of ions - a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-). These ions are then attracted to each other due to electrostatic forces, forming an ionic bond that holds the compound together.
Ionic Bonding in Sodium Chloride
The ionic bonding in sodium chloride is responsible for its crystalline structure, where each sodium ion is surrounded by six chloride ions, and each chloride ion is surrounded by six sodium ions. This arrangement is known as a face-centered cubic lattice structure and contributes to the stability and hardness of the salt crystal. The strong electrostatic attraction between the positively charged sodium ions and the negatively charged chloride ions requires significant energy to overcome, which explains why sodium chloride has a high melting point (800.7°C) and boiling point (1413°C).
| Property | Value |
|---|---|
| Molecular Formula | NaCl |
| Molecular Weight | 58.44 g/mol |
| Melting Point | 800.7°C |
| Boiling Point | 1413°C |

Chemical Properties of Salt

Beyond its composition, the chemical properties of salt are significant in understanding its behavior and applications. Salt is neutral in terms of pH; when dissolved in water, it does not significantly alter the pH of the solution, as neither the sodium ion nor the chloride ion reacts with water to produce acidic or basic compounds. Furthermore, salt is a good conductor of electricity when dissolved in water, due to the presence of sodium and chloride ions that can move freely, carrying electrical charges.
Applications of Salt
Salt has numerous applications across various industries, including culinary, pharmaceutical, and industrial sectors. In the food industry, salt is used as a seasoning and as a preservative to inhibit the growth of bacteria. In the pharmaceutical industry, salt is used in the manufacture of certain drugs. Industrially, salt is used in the production of chlorine and sodium hydroxide (caustic soda), which are essential chemicals in many manufacturing processes.
- Culinary: Seasoning and preservation of food.
- Pharmaceutical: Ingredient in some drugs.
- Industrial: Production of chlorine and sodium hydroxide.
What is the primary composition of salt?
+Salt is primarily composed of sodium chloride (NaCl), making it a compound.
What type of bond is formed in sodium chloride?
+The bond formed in sodium chloride is an ionic bond, resulting from the transfer of electrons between sodium and chlorine atoms.
In conclusion, salt, or sodium chloride, is indeed a compound, consisting of sodium and chlorine atoms chemically bonded together through ionic bonding. Its chemical properties, such as its solubility in water and its role as a conductor of electricity when dissolved, contribute to its wide range of applications. Understanding the composition and properties of salt provides insights into its significance in both natural processes and industrial applications.



