Constant Temperature Process

A constant temperature process is a thermodynamic process in which the temperature of the system remains constant throughout the process. This type of process is also known as an isothermal process. In an isothermal process, the system is in thermal equilibrium with its surroundings, and the heat transfer between the system and the surroundings is reversible. The constant temperature process is a fundamental concept in thermodynamics and has numerous applications in various fields, including physics, chemistry, and engineering.
Characteristics of Constant Temperature Process
A constant temperature process has several distinct characteristics. Firstly, the temperature of the system remains constant, which means that the internal energy of the system does not change. Secondly, the heat transfer between the system and the surroundings is reversible, which implies that the entropy change of the system is equal to the entropy change of the surroundings. Thirdly, the work done by the system is equal to the heat transferred to the surroundings, which is a consequence of the first law of thermodynamics. Finally, the constant temperature process is a quasi-static process, meaning that it occurs slowly and incrementally, allowing the system to remain in equilibrium with its surroundings at all times.
Equation of State for Constant Temperature Process
The equation of state for an ideal gas undergoing a constant temperature process is given by the ideal gas law: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. Since the temperature is constant, the equation of state can be written as P1V1 = P2V2, where the subscripts 1 and 2 refer to the initial and final states of the process. This equation shows that the product of the pressure and volume of an ideal gas remains constant during an isothermal process.
| Quantity | Initial State | Final State |
|---|---|---|
| Temperature (T) | T1 | T2 = T1 |
| Pressure (P) | P1 | P2 |
| Volume (V) | V1 | V2 |
| Internal Energy (U) | U1 | U2 = U1 |
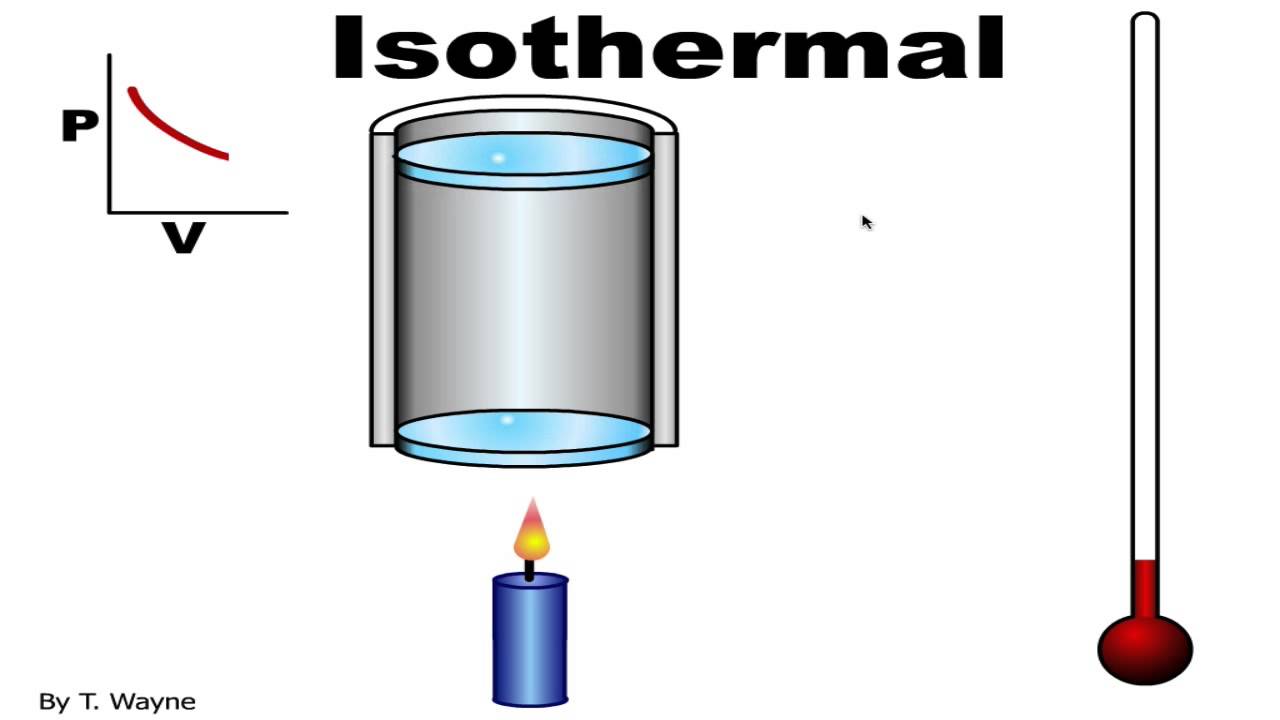
Applications of Constant Temperature Process
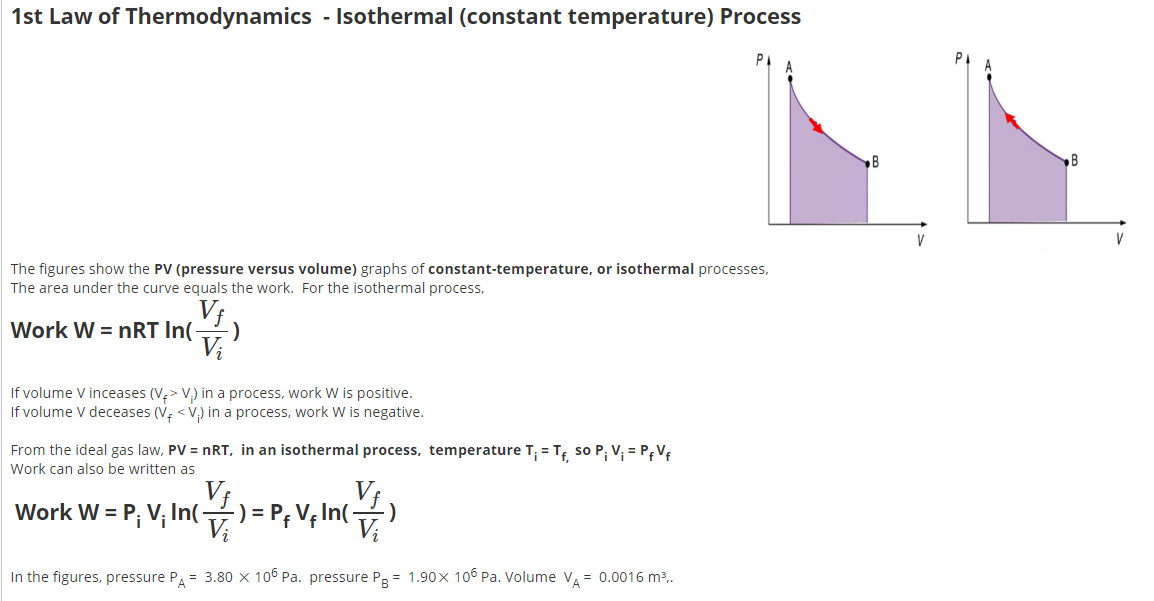
The constant temperature process has numerous applications in various fields, including physics, chemistry, and engineering. One of the most significant applications is in the field of refrigeration, where the isothermal process is used to transfer heat from a cold body to a hot body. The constant temperature process is also used in the production of liquefied gases, such as oxygen and nitrogen, where the gas is cooled to a very low temperature at constant pressure. Additionally, the isothermal process is used in the design of heat engines, such as internal combustion engines and steam engines, where the goal is to maximize the efficiency of the engine by minimizing the heat transfer between the system and the surroundings.
Types of Constant Temperature Processes
There are several types of constant temperature processes, including expansion, compression, and cyclic processes. In an expansion process, the system expands against a constant external pressure, resulting in an increase in volume and a decrease in pressure. In a compression process, the system is compressed by a constant external pressure, resulting in a decrease in volume and an increase in pressure. In a cyclic process, the system undergoes a series of expansion and compression processes, resulting in a closed loop on a pressure-volume diagram.
- Expansion process: The system expands against a constant external pressure, resulting in an increase in volume and a decrease in pressure.
- Compression process: The system is compressed by a constant external pressure, resulting in a decrease in volume and an increase in pressure.
- Cyclic process: The system undergoes a series of expansion and compression processes, resulting in a closed loop on a pressure-volume diagram.
What is the difference between an isothermal process and an adiabatic process?
+An isothermal process is a thermodynamic process in which the temperature of the system remains constant, whereas an adiabatic process is a thermodynamic process in which there is no heat transfer between the system and the surroundings. In an adiabatic process, the temperature of the system may change, whereas in an isothermal process, the temperature remains constant.
What is the equation of state for an ideal gas undergoing a constant temperature process?
+The equation of state for an ideal gas undergoing a constant temperature process is given by the ideal gas law: PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. Since the temperature is constant, the equation of state can be written as P1V1 = P2V2, where the subscripts 1 and 2 refer to the initial and final states of the process.
In conclusion, the constant temperature process is a fundamental concept in thermodynamics, with numerous applications in various fields. The equation of state for an ideal gas undergoing an isothermal process provides a useful tool for analyzing and understanding the behavior of the system. The different types of constant temperature processes, including expansion, compression, and cyclic processes, have distinct characteristics and applications. By understanding the constant temperature process, we can gain insights into the behavior of thermodynamic systems and design more efficient and effective systems for various applications.