Citric Acid Formula
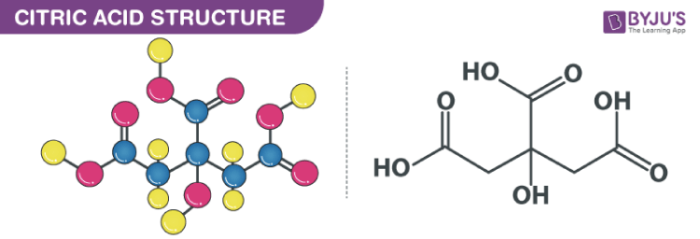
Citric acid is a naturally occurring compound that is widely used in various industries, including food, pharmaceutical, and cosmetics. The chemical formula for citric acid is C6H8O7, indicating that it consists of six carbon atoms, eight hydrogen atoms, and seven oxygen atoms. This formula is essential in understanding the properties and behavior of citric acid in different applications.
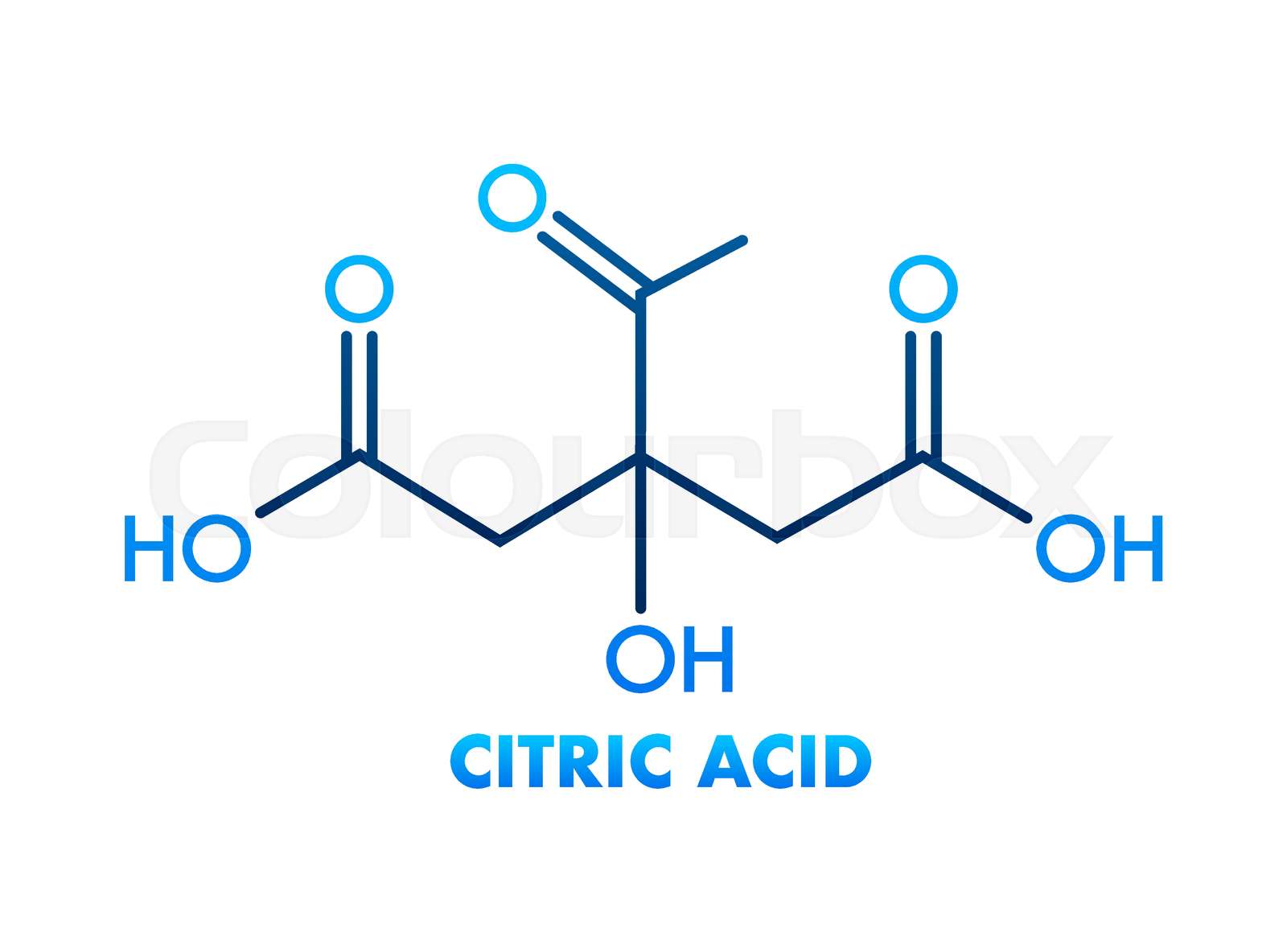
Citric Acid Structure and Properties

Citric acid is a weak organic acid that is highly soluble in water. Its molecular structure consists of a central carbon atom bonded to three carboxyl groups (-COOH) and a hydroxyl group (-OH). This unique structure allows citric acid to form complexes with metals and other compounds, making it a useful chelating agent. The molecular weight of citric acid is 192.12 g/mol, and its density is approximately 1.66 g/cm3 at room temperature.
Citric Acid Chemical Reactions
Citric acid undergoes various chemical reactions, including neutralization, esterification, and fermentation. When citric acid reacts with a base, such as sodium hydroxide (NaOH), it forms a salt and water. This reaction is commonly used in the production of citrate salts, which are used as food preservatives and pharmaceutical excipients. The reaction equation for the neutralization of citric acid is: C6H8O7 + 3NaOH → C6H5Na3O7 + 3H2O.
| Physical Property | Value |
|---|---|
| Molecular Weight | 192.12 g/mol |
| Density | 1.66 g/cm3 |
| Melting Point | 153°C |
| Boiling Point | 230°C (decomposition) |

Citric Acid Production and Applications
Citric acid is produced through microbial fermentation, where microorganisms such as Aspergillus niger convert sugars into citric acid. The resulting citric acid is then purified and concentrated through various methods, including crystallization and distillation. Citric acid is used in various applications, including food and beverages, pharmaceuticals, and cosmetics. In the food industry, citric acid is used as a preservative and flavor enhancer, while in the pharmaceutical industry, it is used as an excipient and chelating agent.
Citric Acid in Food and Beverages
Citric acid is widely used in the food and beverage industry due to its unique properties. It is used as a preservative to extend the shelf life of food products, and as a flavor enhancer to add a sour taste to beverages. Citric acid is also used in the production of soft drinks, fruit juices, and energy drinks. The concentration of citric acid in food products can vary, but it is typically used at levels between 0.1% and 1.0%.
- Citric acid is used in the production of soft drinks, such as lemon-lime soda and orange juice.
- Citric acid is used as a preservative in food products, such as jams, jellies, and marmalades.
- Citric acid is used as a flavor enhancer in beverages, such as energy drinks and sports drinks.
What is the chemical formula for citric acid?
+The chemical formula for citric acid is C6H8O7.
What are the main applications of citric acid?
+Citric acid is used in various applications, including food and beverages, pharmaceuticals, and cosmetics. It is used as a preservative, flavor enhancer, excipient, and chelating agent.