Atomic Size Periodic Table: Easy Element Lookup

The atomic size periodic table is a valuable tool for chemists, physicists, and researchers, providing a comprehensive overview of the elements and their properties. The periodic table is a tabular arrangement of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. In this article, we will delve into the world of atomic size and explore how the periodic table can be used for easy element lookup.
Understanding Atomic Size
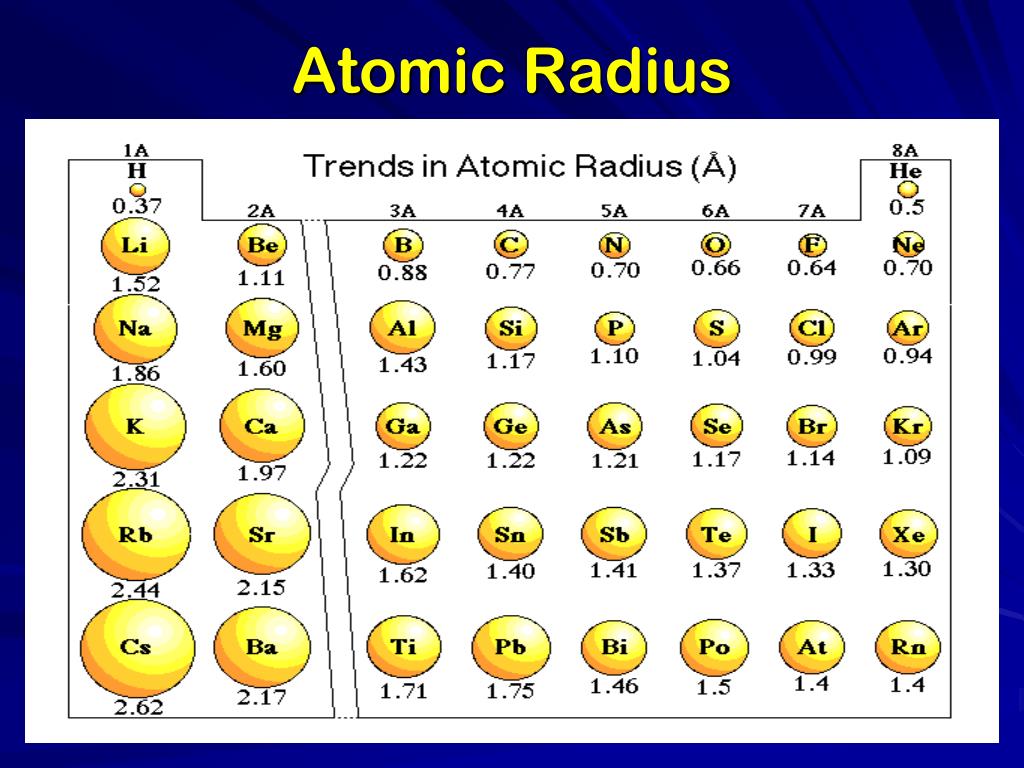
Atomic size, also known as atomic radius, is a measure of the size of an atom, typically defined as the distance from the nucleus to the outermost electron. The atomic size of an element is influenced by the number of protons in the nucleus, the number of electron shells, and the effective nuclear charge. As we move across a period in the periodic table, the atomic size decreases due to the increasing effective nuclear charge, which pulls the electrons closer to the nucleus. In contrast, as we move down a group, the atomic size increases due to the addition of new electron shells, which results in a greater distance between the nucleus and the outermost electrons.
Factors Affecting Atomic Size
Several factors affect the atomic size of an element, including:
- Electron configuration: The arrangement of electrons in an atom, which determines the size of the atom.
- Nuclear charge: The positive charge of the nucleus, which attracts the electrons and affects the size of the atom.
- Electron shielding: The phenomenon where inner electrons shield the outer electrons from the nuclear charge, reducing the effective nuclear charge and increasing the atomic size.
- Orbital shape: The shape of the atomic orbitals, which affects the size of the atom.
| Element | Atomic Number | Atomic Radius (pm) |
|---|---|---|
| Hydrogen | 1 | 37 |
| Helium | 2 | 32 |
| Lithium | 3 | 152 |
| Beryllium | 4 | 112 |

Periodic Table Trends
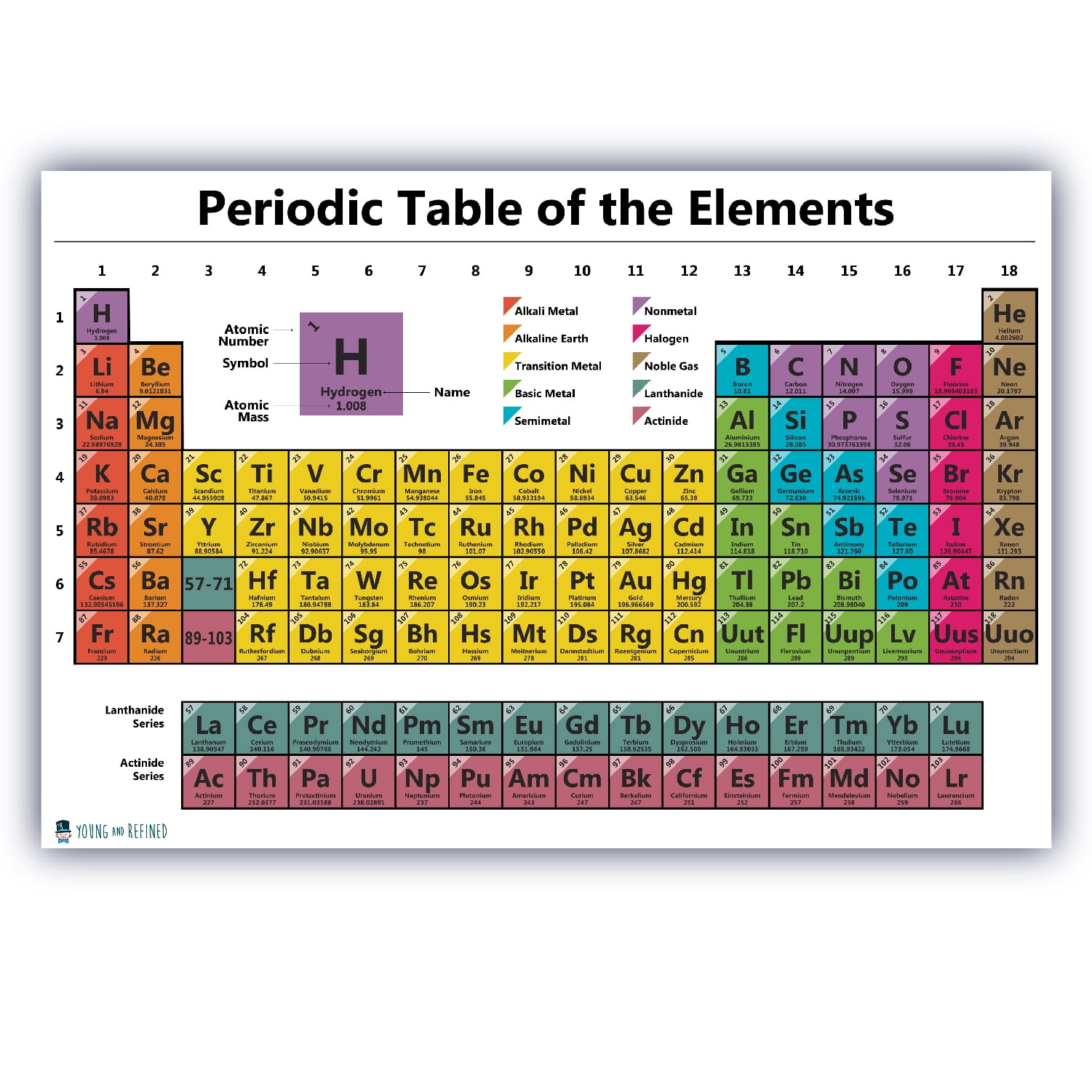
The periodic table exhibits several trends, including:
The atomic size trend is one of the most significant trends in the periodic table. As we move across a period, the atomic size decreases, while as we move down a group, the atomic size increases. This trend is a result of the changing effective nuclear charge and the addition of new electron shells.
Group Trends
Each group in the periodic table exhibits a unique set of trends, including:
- Alkali metals (Group 1): These elements have the largest atomic size in their respective periods and exhibit a strong tendency to lose one electron to form a positive ion.
- Noble gases (Group 18): These elements have a full outer energy level and exhibit a low reactivity due to their stable electronic configuration.
- Halogens (Group 17): These elements have a high electronegativity and exhibit a strong tendency to gain one electron to form a negative ion.
What is the significance of atomic size in the periodic table?
+The atomic size of an element is a critical parameter in understanding its chemical properties and behavior. By analyzing the atomic size of an element, researchers can gain insights into its reactivity, solubility, and potential applications.
How does the atomic size change across a period in the periodic table?
+As we move across a period in the periodic table, the atomic size decreases due to the increasing effective nuclear charge, which pulls the electrons closer to the nucleus.
In conclusion, the atomic size periodic table is a powerful tool for understanding the properties and behavior of elements. By analyzing the trends and patterns in the periodic table, researchers can gain insights into the chemical properties and potential applications of elements. Whether you are a student, researcher, or industry professional, the atomic size periodic table is an essential resource for easy element lookup and exploration.