Atomic Number Of Neon

The atomic number of neon is a fundamental concept in chemistry and physics, and it plays a crucial role in understanding the properties and behavior of this element. Neon is a noble gas with the symbol Ne and an atomic number of 10. This means that a neon atom has 10 protons in its nucleus, which defines its chemical properties and determines its position in the periodic table.
Introduction to Atomic Number
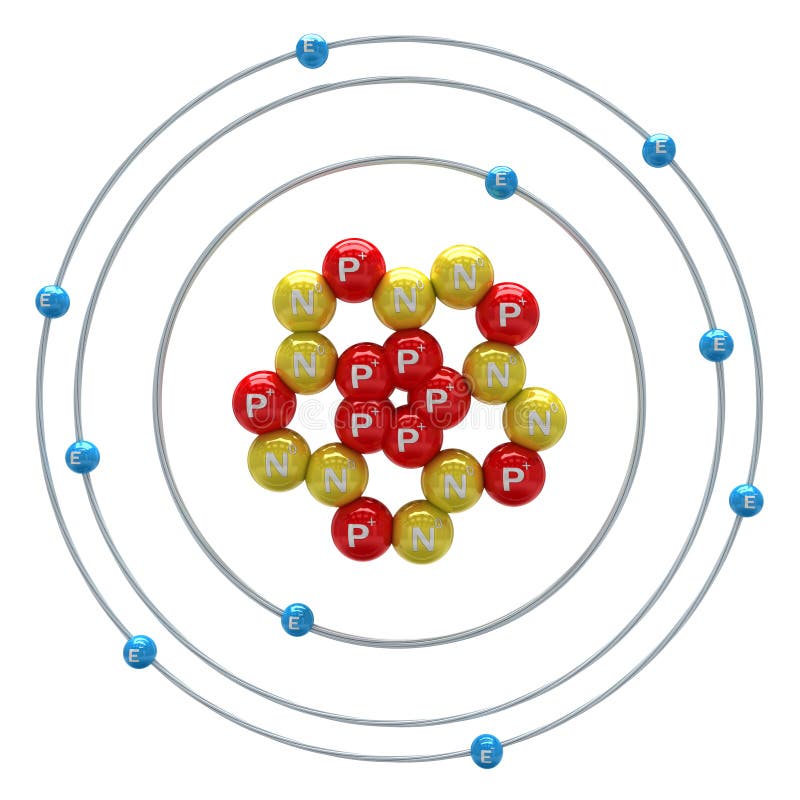
The atomic number is a unique identifier for each element and is defined as the number of protons present in the nucleus of an atom. It is a fundamental property of an element that determines its chemical behavior, electron configuration, and position in the periodic table. The atomic number of an element is always a whole number and is denoted by the symbol Z. In the case of neon, its atomic number is 10, which means that it has 10 protons in its nucleus.
Electronic Configuration of Neon
The electronic configuration of neon is 1s² 2s² 2p⁶, which means that it has a full outer energy level. This configuration is responsible for the stability and unreactivity of neon, as it has a full octet of electrons in its outermost energy level. The electronic configuration of neon is also responsible for its chemical properties, such as its inability to form compounds with other elements.
| Element | Symbol | Atomic Number | Electronic Configuration |
|---|---|---|---|
| Neon | Ne | 10 | 1s² 2s² 2p⁶ |

Properties of Neon
Neon is a noble gas with a number of unique properties that make it useful in a variety of applications. It is a colorless, odorless, and tasteless gas that is lighter than air. Neon is also an excellent insulator and has a high ionization energy, which makes it difficult to ionize. These properties make neon useful in applications such as lighting, lasers, and refrigeration.
Uses of Neon
Neon has a number of uses due to its unique properties. It is used in neon signs, which are used for advertising and decoration. Neon is also used in plasma TVs and other display devices, where it is used to create the images on the screen. Additionally, neon is used in lasers, where it is used to create high-intensity beams of light. Neon is also used in refrigeration, where it is used as a refrigerant due to its low boiling point and high heat transfer coefficient.
- Neon signs
- Plasma TVs
- Lasers
- Refrigeration
What is the atomic number of neon?
+The atomic number of neon is 10, which means that it has 10 protons in its nucleus.
What is the electronic configuration of neon?
+The electronic configuration of neon is 1s² 2s² 2p⁶, which means that it has a full outer energy level.
What are some uses of neon?
+Neon is used in a variety of applications, including neon signs, plasma TVs, lasers, and refrigeration.