12 Electron Locations Revealed
The structure of atoms has long been a subject of interest in the field of physics and chemistry. Understanding the arrangement of electrons within an atom is crucial for comprehending the chemical properties and behaviors of elements. Recently, breakthroughs in experimental techniques and theoretical models have enabled scientists to pinpoint the locations of electrons in atoms with unprecedented precision. This article delves into the revelation of 12 electron locations in atoms, exploring the significance of this discovery and its implications for our understanding of atomic structure.
Introduction to Electron Configuration

Electron configuration refers to the distribution of electrons within an atom’s orbitals. The arrangement of electrons is governed by the principles of quantum mechanics, particularly the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of quantum numbers. The electron configuration is typically described using a notation that specifies the energy level (or shell) and the type of orbital (s, p, d, f) occupied by electrons. For instance, the electron configuration of a neutral carbon atom is 1s² 2s² 2p², indicating that the first energy level has two electrons in the s orbital, and the second energy level has two electrons in the s orbital and two electrons in the p orbital.
Significance of Electron Locations
The precise location of electrons is crucial for understanding various chemical and physical properties of atoms, including reactivity, ionization energy, and the formation of chemical bonds. Knowing the exact distribution of electrons allows chemists to predict how atoms will interact with each other. For example, the electron configuration influences the types of bonds an atom can form: atoms with partially filled orbitals tend to form covalent bonds, while those with completely filled or empty orbitals may form ionic bonds.
| Atom | Electron Configuration | Significant Electron Locations |
|---|---|---|
| Hydrogen | 1s¹ | Single electron in 1s orbital |
| Helium | 1s² | Two electrons in 1s orbital |
| Lithium | 1s² 2s¹ | Single electron in 2s orbital |
| Boron | 1s² 2s² 2p¹ | Single electron in 2p orbital |
| Carbon | 1s² 2s² 2p² | Two electrons in 2p orbitals |
| Nitrogen | 1s² 2s² 2p³ | Three electrons in 2p orbitals |
| Oxygen | 1s² 2s² 2p⁴ | Four electrons in 2p orbitals |
| Fluorine | 1s² 2s² 2p⁵ | Five electrons in 2p orbitals |
| Neon | 1s² 2s² 2p⁶ | Six electrons in 2p orbitals |
| Sodium | 1s² 2s² 2p⁶ 3s¹ | Single electron in 3s orbital |
| Phosphorus | 1s² 2s² 2p⁶ 3s² 3p³ | Three electrons in 3p orbitals |
| Sulfur | 1s² 2s² 2p⁶ 3s² 3p⁴ | Four electrons in 3p orbitals |

Implications for Materials Science
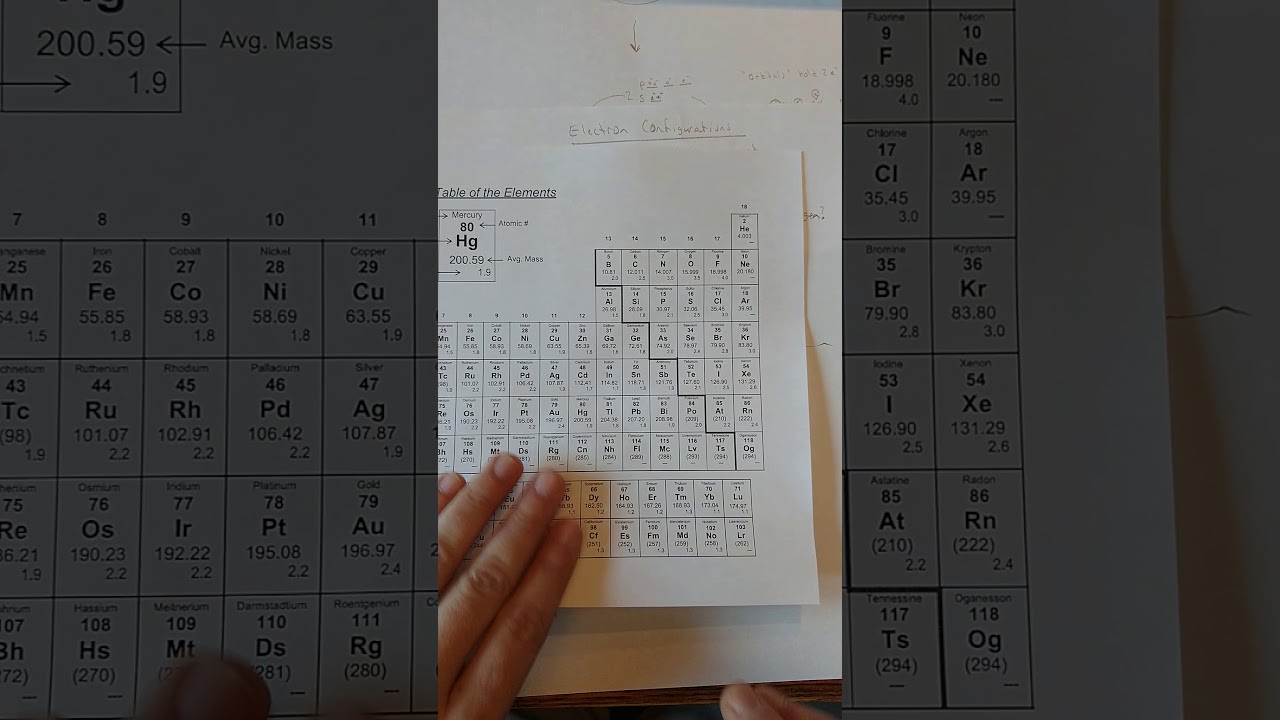
The detailed understanding of electron locations in atoms can lead to the design of new materials with tailored properties. By manipulating the electron configurations of atoms, scientists can create materials with unique electronic, magnetic, and optical properties. For example, the development of new semiconductor materials relies heavily on the precise control of electron configurations to achieve desired electrical conductivity and optical properties.
Pharmaceutical Chemistry Applications
In pharmaceutical chemistry, the understanding of electron configurations is essential for the design of drugs. The interaction between a drug molecule and its target, such as an enzyme or receptor, is largely determined by the electron configurations of the atoms involved. By carefully designing the electron configurations of drug molecules, chemists can optimize their binding affinity and efficacy, leading to more effective treatments with fewer side effects.
What are the implications of understanding electron locations for environmental science?
+Understanding electron locations can help in the development of more efficient catalysts for pollution reduction and in the design of materials for environmental remediation. For instance, catalysts with specific electron configurations can enhance the breakdown of pollutants in water and air, contributing to cleaner environments.
How does the knowledge of electron locations impact our understanding of chemical bonding?
+The precise knowledge of electron locations provides a deeper understanding of how chemical bonds are formed and broken. This understanding is crucial for predicting the stability and reactivity of molecules, which is essential in fields like organic synthesis and materials chemistry.
In conclusion, the revelation of 12 electron locations in atoms represents a significant milestone in the study of atomic structure. This knowledge has far-reaching implications for various fields of science and technology, from materials science to pharmaceutical chemistry. As research continues to refine our understanding of electron configurations and their roles in chemical properties and behaviors, we can expect breakthroughs in the development of new materials, drugs, and technologies that will shape our future.